
KTL to Present at MBAA District Carolinas Technical Conference
KTL will be joining industry experts at the 2024 Master Brewers Association of America’s (MBAA) District Carolina Technical Conference to discuss food safety and regulatory implications of producing nonalcoholic and low-alcohol beer (NALAB).
MBAA District Carolinas 2024 Spring Technical Conference
May 4, 2024
Hopfly Brewing Company | Charlotte, NC
KTL Presentation: Food Safety and Regulatory Implications of Producing Nonalcoholic and Low-Alcohol Beer (NALAB)
The demand for NALAB has increased significantly in the past few years and is projected to continue. KTL’s presentation will describe the complexities associated with adding a NALAB beverage to a product portfolio and break down the requirements and considerations into actionable pieces. It will also discuss relevant food safety risks in traditional beer, such as allergen cross-contamination, chemical inclusion, and foreign object inclusion. Specifically, the presentation will cover the following:
- Interpretation and breakdown of the regulatory requirements for NALAB products.
- Differences in FDA regulatory requirements for traditional beer versus NALAB products.
- Known or reasonably foreseeable food safety hazards associated with traditional beer and NALAB products.
- Strategies that brewers can take to minimize food safety hazards.

Don’t Miss KTL at the 2024 Food Safety Summit
As one of the premier events in the food industry, the Food Safety Summit provides a comprehensive conference and expo for attendees to learn from subject matter experts, exchange ideas, and find solutions to current industry challenges.
- When: May 6-9, 2024
- Where: Donald Stephens Convention Center, Rosemont, Illinois
- Who: Retailers, food processors, distributors, food manufacturers, growers, foodservice, testing laboratories, importing/exporting, law firms, and other food safety professionals
- Find KTL: Stop by our booth (#406) in the exhibit hall!
Tech Tent Case Study Presentation
Be sure to also update your agenda to attend KTL’s Tech Tent presentation on Thursday, May 9 at 11:15 am CT:
Case Study: Leveraging Existing Microsoft® Software to Develop a Robust FSMS
KTL will present a case study demonstrating how Italian Frozen Foods (IFF) USA is leveraging the company’s existing software—Microsoft Power Platform with SharePoint®—to elevate its food safety management system (FSMS) and effectively manage food safety compliance documentation, data, and certification requirements.
- Learn how you can use the software you already have to develop a robust FSMS.
- Understand the approach and process required to ensure successful development and implementation.
- Observe in practice how an integrated system can help organize, control, analyze, and visualize information so you remain in compliance and operate more efficiently.

FAQs on ISO’s New Climate Change Amendments
Effective February 23, 2024, the International Organization for Standardization (ISO) is integrating climate change considerations into all management system standards through its Climate Change Amendments. These Amendments ensure climate change impacts are considered by all organizations in their management system design and implementation.
ISO’s recent action supports the London Declaration on Climate Change of September 2021, which establishes ISO’s commitment to combatting climate change through its standards and publications. The aim of the recent Amendments is to make climate change an integral part of management systems design and implementation to help guide organizational strategy and policy.
What are the changes?
The Climate Change Amendments explicitly require climate change considerations in all existing and future ISO management systems standards, as incorporated into the Harmonized Structure (Appendix 2 of the Annex SL in the ISO/IEC Directives Part 1 Consolidated ISO Supplement). More specifically, the Amendments add the following two new statements to Annex SL for organizations to consider the effects of climate change on the management system’s ability to achieve its intended results:
- Clause 4.1: The organization shall determine whether climate change is a relevant issue, as it relates to understanding the organization and its context.
- Clause 4.2: NOTE: Relevant interested parties can have requirements related to climate change, pertaining to understanding the needs and expectations of interested parties.
The broad scope of these Amendments (i.e., impacting all standards) reflects ISO’s commitment to integrating climate considerations across diverse operational areas (e.g., environment, quality, safety, food safety, security, business continuity, etc.).
What do these changes require?
The original intent and requirements of Clauses 4.1 and 4.2 remain unchanged; however, the Amendments now require organizations to consider the relevance of climate change risks and impacts on the management system(s).
Potential climate change issues will likely differ for the various standards. The Amendments ensure these various risks are considered for each standard and, if actions are required, allow the organization to effectively plan for them in the management system.
What do certified organizations need to do?
Organizations that are certified—or are planning for certification—need to make sure they consider climate change aspects and risks in the development, maintenance, and effectiveness of their management system(s).
The Amendments specifically require these organizations to evaluate and determine whether climate change is a relevant issue within their management system(s). If the answer is yes, the organization then must consider climate change in a risk evaluation within the scope of their management systems. Where relevant, organizations are further encouraged to integrate climate change into their strategic objectives and risk mitigation efforts. The Amendments do not require organizations to do anything about climate change beyond considering the impacts on the management system’s ability to achieve its intended results.
What is the timeline?
The Amendments are effective as of the date of publication. There is no transition for implementation.
Certification bodies and auditors will cover the Amendments in audit activities when assessing this section of a management system. The audit will ensure climate change is considered and, if determined to be a relevant issue, included in company objectives and risk mitigation efforts. If climate change is deemed not relevant, the audit will assess the organization’s process for making this determination.
Will new certifications be issued?
Because the changes are considered a clarification, ISO issued them in the form of an amendment. New standards will not be republished until new versions are released; therefore, the publication year of each ISO standard will not change, and no new certifications will be issued.
What are the benefits of these changes?
The Climate Change Amendments underscore the importance of understanding and addressing the impacts of climate change. By publishing the Amendments in Annex SL, ISO is leveraging the widespread adoption of all ISO management system standards across operational areas to integrate environmental stewardship into organizational practices, promote sustainability, and drive climate change action on a global scale.
For certified organizations, the Amendments are intended to enhance organizational resilience and adaptability to climate-related risks. Considering climate change in this way can significantly contribute to business sustainability and long-term success by:
- Ensuring regulatory compliance (e.g., emission limits, sustainability reporting, etc.).
- Creating positive brand reputation as a sustainable company and associated customer loyalty.
- Managing risks and opportunities associated with supply chain disruptions, energy efficiency initiatives, employee health and safety, natural disasters, etc.
- Engaging employees and attracting new talent who prioritizes sustainability.
- Providing access to markets and investors that have sustainability requirements.

Comments: No Comments
Focus on Employee Safety in the Food Industry
Safety hazards exist in every manufacturing environment. The food industry is no exception. Between October 2018 and September 2019, the Occupational Safety and Health Administration (OSHA) issued a total of 1,168 citations resulting in over $7 million in fines to the food manufacturing industry alone.
Occupational safety and health risks in food manufacturing are often heightened because of the nature of the product (i.e., food or drink) being manufactured. Even food safety measures taken to prevent contamination and ensure food safety can carry inherent occupational safety and health risks.
While food safety is paramount for any company operating in the food industry, a company cannot stay in business if they do not take the appropriate measures to keep employees healthy and safe.
Common Safety Hazards in the Food Industry
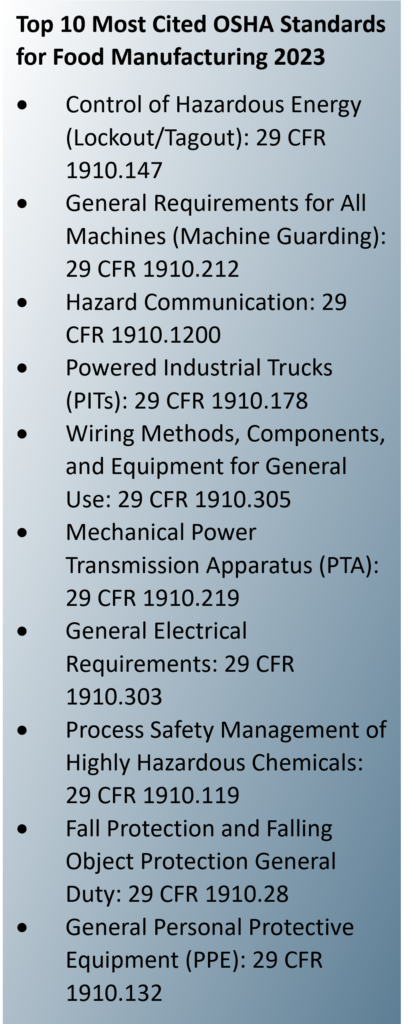
Lockout/tagout and machine guarding recurringly top OSHA’s annual list of the most frequently cited standards in food manufacturing. In fact, the most expensive OSHA fines of 2023 involved two food manufacturers and violations concerning machine guarding and lockout/tagout.
Lockout/Tagout
Employees need to be protected against the unexpected startup or release of energy. Lockout/tagout involves properly de-energizing and securing equipment so it cannot be operated unsafely when a machine needs service or repair. When employees work in a fast-paced environment, they may not take the required steps to first properly de-energize the equipment. As a result, approximately 1,000 workers die each year due to unexpected operation of equipment and/or release of stored energy. Machines and electrical equipment must be properly shut down, de-energized, and locked when servicing.
Machine Guarding
Moving machine parts have the potential to cause severe workplace injuries, such as crushed fingers or hands, amputations, burns, blindness—and most food processing machinery includes pinch points (i.e., blades, rolling parts, presses, etc.) that can put workers at increased risk. Safeguards for machine parts, functions, and processes are essential for controlling hazards and protecting workers from these otherwise preventable injuries.
Other Common Safety Hazards
- Ergonomics. Many food manufacturing jobs involve repetitive motion that can cause musculoskeletal disorders.
- Slips, trips, and falls. Sticky or wet products and frequent cleaning can both contribute to slippery work surfaces that increase the risk for slips, trips, and falls. The high volume of liquids used in food manufacturing and processing creates regular employee exposure to wet surfaces.
- Chemicals and harmful substances. Food facilities rely on various chemical sanitizers and disinfectants to prevent contamination. In addition, anhydrous ammonia, a common refrigerant used in food facilities, is very hazardous (i.e., corrosive, flammable, and explosive), even in small spaces.
- Cut hazards. Knives and other blades are common equipment in food processing plants. Dull blades cause more accidents because they are harder to work with, require more pressure, and may slip more easily. Blades should be sharpened, and employees should wear appropriate PPE.
OSHA Response: Local Emphasis Programs
Between 2016 and 2020, OSHA investigated fatalities, amputations, fractures, and crushed hands and fingers at food manufacturing facilities and identified the primary causes as failure to control hazardous energy or implement adequate machine guarding. In Wisconsin, Bureau of Labor and Statistics (BLS) data from 2011-2020 show that food manufacturing injury rates were consistently higher than the averages for all Wisconsin manufacturing companies. In 2019, OSHA found that food production workers in Ohio had a nearly 57% higher rate of amputations and 16% higher rate of fractures; in Illinois, these rates were 29% higher for amputations and 14% higher for fractures compared to private sector manufacturing.
In 2022, OSHA Region 5 established two Local Emphasis Programs (LEPs)—one for Wisconsin and one for Illinois and Ohio— to encourage employers to identify, reduce, and eliminate exposure to machine hazards during production activities and off-shift sanitation, service, and maintenance tasks (i.e., machine guarding and hazardous energy control – lockout/tagout). These LEPs run through at least 2027 and focus on reducing fatalities and injuries through outreach, education, training, and enforcement activities.
The LEP empowers OSHA to schedule and inspect food industry employers whose injury rates exceed the state average among all manufacturers. The scope of inspections conducted under the LEP focus on reviewing:
- Production operations, sanitation processes, and working conditions.
- Injury and illness records (i.e., OSHA 300 logs), particularly injuries pointing to deficiencies in machine guarding or the hazardous energy control program.
- Machine guarding hazards associated with points of operation, ingoing nip points, and moving or rotating parts of food processing equipment.
- Deficiencies in the hazardous energy control program associated with equipment service, maintenance, setup, and sanitation.
- Hazards associated with chemical burns from corrosives, such as those used for cleaning and sanitizing.
Prepare Your Facility…Protect Your Workers
If you fall under an OSHA LEP, you need to prepare your facility. Even if you don’t, you need to protect your workers.
- Prepare and maintain your OSHA 300 log with any work-related injuries and illnesses. KTL’s OSHA 300 PowerApp can make it easier to collect, search, analyze, store, and aggregate data so it is available when needed.
- Conduct a thorough hazard analysis of the facility, operations, and processes to identify potential safety hazards. The hazard analysis should answer what could potentially go wrong, what the associated consequences are, and how they can be prevented or eliminated.
- Develop, implement, and maintain the appropriate safety programs, procedures, and instructions. A safety management system that aligns with current food safety systems can provide resources to help companies identify and manage safety risks and an organizing framework for policies, procedures, and practices, including:
- Engineering controls for dangerous equipment, including machine guarding.
- Written energy control program and lockout/tagout procedures specific to each piece of equipment.
- Emergency response programs.
- Proper maintenance, cleaning, and sanitation procedures and schedules.
- Guidelines for proper use, care, and replacement of PPE.
- Train your staff. Workers need to have appropriate training, including use of PPE, hazards of extreme temperatures, material handling, hazard communication, lockout/tagout procedures, machine guarding procedures, etc. Initial safety training should be conducted when onboarding new employees, with refresher training provided annually to ensure competency. Training tracking systems allow for the centralized implementation, management, tracking, scheduling, assignment, and analysis of organizational training efforts.
- Provide proper PPE. Workers should be wearing proper footwear, gloves, safety glasses, ear plugs, aprons, etc. and be provided with anti-slip mats to ensure safety.
- Ensure safety data sheets (SDS) are current and available to help identify hazardous chemicals and protect employees from exposure. Train workers on the chemicals used in the workplace, including first aid, what to do in the event of a release, identifying characteristics, proper procedures for working around or with the chemical, and appropriate PPE.
- Use visual communication (e.g., labels, signage) to help protect workers from hot surfaces, exposed moving parts, pressurized systems, hazardous chemicals, slippery surfaces, and more.
Finally, prioritize safety. Small steps can go a long way in making sure employees leave work safely versus becoming an OSHA statistic.

Comments: No Comments
Challenging the Food Defense Program
The Food and Drug Administration’s (FDA) Intentional Adulteration (IA) Rule aims to prevent acts targeting the food supply that are intended to cause wide-scale harm to public health. The IA Rule requires food businesses to assess and identify vulnerabilities in their processes, operations, and facilities; implement strategies to reduce those vulnerabilities; and then regularly monitor and verify the effectiveness of those strategies to reduce the occurrence of IA acts. A food defense “challenge” (also known as an intrusion test) is then used to assess how well those strategies are implemented throughout the organization.
What is the purpose of the IA intrusion test?
The purpose of an intrusion test is to evaluate a company’s existing food defense programs for proper implementation across the company by “challenging” the system’s efficacy. In an unannounced intrusion test, a food safety expert scopes out the facility and its vulnerabilities, attempts to gain access to sensitive areas, and tests how the food defense system works in practice.
The test helps facilities to:
- Assess whether food defense procedures are effectively implemented throughout the facility—and are being followed by personnel.
- Highlight facility strengths and implementation failures.
- Close identified gaps and reinforce programs, where needed.
- Meet FDA regulatory and Global Food Safety Initiative (GFSI) certification standard requirements.
Am I required to do one of these challenges?
To truly ensure food safety and defense programs work, they need to be challenged. The GFSI benchmarked certification standards all require some version of testing:
- SQF: The food defense program must be tested to ensure food defense strategies are effective, appropriate, and documented. This test can be based on a fictitious scenario that allows an intruder to enter the building and challenge the system.
- FSSC 22000: Organizations must conduct and document a food defense threat assessment based on defined methodology to identify and evaluate potential threats linked to processes and products.
- BRCGS: A documented risk assessment must be conducted to identify potential risks to the security of product and to ensure appropriate controls implemented to reduce the risk.
- IFS: The food defense plan must be tested for effectiveness and reviewed at least annually or whenever significant changes occur.
What does the process look like?
An important part of these tests is that they are unannounced, so personnel continue to operate as usual versus being on heightened alert. Tests are typically coordinated and scheduled with company leadership to take place at a time/date that is undisclosed to plant employees. Company leadership provides the food safety expert with documentation that confirms the test and outlines who to contact when/if “apprehended” to help keep the food safety expert safe.
Prior to conducting the test, the food safety expert will generally tour the facility to identify sensitive areas to target and opportunities to enter the facility. This is important since the first critical step in conducting the test involves gaining access to the building, whether through finding unlocked doors, pretending to be plant personnel, or even creating a cover story and walking through the front door.
Once the food safety expert is in the facility, the objective becomes to try to access as many sensitive areas as possible to truly “test” the system. The intrusion test is followed up with a detailed findings report that provides recommendations for closing gaps and strengthening the facility’s food defense program.
What does the IA intrusion test include?
An IA internal intrusion test evaluates company preparedness for intentional trespassing and internal attacks on sensitive areas inside the facility. The intruder will take advantage of employee movement (e.g., arrivals, breaks, lunches, departures); focus on physical controls of building access (e.g., employee entrances, garage doors, receiving/loading areas); and target sensitive internal areas within the building. The challenge will involve testing facility procedures and employee implementation, as well as the following food defense mechanisms:
- Card access/badge system/key protocol
- Reception area
- Visitor control/sign-in program
- Security guards
- Camera system
- Exterior door security
- Lighting
- Shipping/receiving areas
- Fencing
- Parking lot
What are common missteps identified in intrusion tests?
In many cases, the issues encountered are seemingly small things; however, the small issues can quickly result in very big problems if an intruder is able to access sensitive areas. Some of these include the following:
- Employees forget to ensure the door is shut and fully latched when leaving an area.
- Employees do not question the presence of visitors and, in some cases, will even open the door for an unknown individual.
- Badges and uniforms are left unattended throughout the facility for intruders to steal and use as identification. With this identification, interior areas are relatively easy to access and contaminate.
- Doors are not locked—either inadvertently or because locking mechanisms are not repaired—or are propped open for ease of access.
- Equipment on the exterior of the facility (e.g., ladders, company vehicles, tools, etc.) is not adequately controlled.
What can facilities do to prevent IA?
Training staff to understand food defense risks, identify suspicious activities and individuals, and implement preventive measures is key to reducing IA risk. FDA and the Department of Homeland Security’s (DHS) See Something, Say Something Campaign reminds front-line employees of what to look for and how to respond if suspicious activity occurs.
Importantly, staff must feel empowered to notify management if they notice anything suspicious. This might include someone taking notes or videos in the plant; an individual attempting to gain access to restricted areas; theft of employee uniforms, badges, or packaging labels; unattended vehicles illegally parked near the facility; unattended items near the business (e.g., boxes, bags); and suspicious work behaviors/hours from employees.
Best practices to prevent this suspicious activity from causing wide-scale harm include the following:
- Post security personnel to serve as a deterrent for intruders.
- Properly maintain exterior doors, parking lots, and outside lighting.
- Keep doors locked and closed, including entrance, break room, and loading docks.
- Ensure visitor stickers are removed and disposed of upon checkout.
- Secure open containers of food/food ingredients.
- Control access of all employees, visitors, contractors, delivery personnel, etc. to sensitive areas.
- Make sure visitors to the facility are accompanied by an employee.
- Monitor products for evidence of tampering (i.e., seals, labels, packaging).
- Complete background checks on employees.
- Train personnel to report suspicious individuals or activities.

Now Hiring: Food Safety Specialist
Location: Chicago, Illinois (other locations considered)
KTL is seeking a Food Safety Specialist with 5-10 years of professional food safety consulting or relevant food industry experience to join our team. This individual will work under the direction of KTL Project Managers and Senior Consultants to manage and execute tasks for KTL’s food safety projects and meet client expectations. The Food Safety Specialist must have working knowledge of FDA, USDA, and GFSI requirements as they apply to food/food packaging manufacturing, processing, and distribution, and experience implementing/maintaining food safety documents and plans.
Responsibilities and tasks include the following:
- Providing HACCP, SOP, and SSOP development and implementation support
- Conducting gap assessments to FDA, USDA, and GFSI (i.e., IFS, BRC, FSSC22000, SQF) requirements
- Conducting relevant food safety training for clients
- Researching FDA, USDA, and GFSI regulatory requirements and maintaining standards updates
- Researching labeling regulatory review
- Interpreting third–party regulatory audits
- Reviewing, recommending, and coordinating efforts for environmental contaminants and pathogen testing program
- Working with clients and KTL senior staff to identify Food Safety Management System (FSMS) and program gaps and implement solutions for continuous improvement
- Maintaining and updating documents to ensure conformance and compliance consistency
- Participating in the development and management of KTL’s SharePoint® tools
- Assisting in growing clients and other business development efforts, as requested
Salary Range: $65,000 to $90,000 per year
Requirements
- B.S. degree in food science, biology, chemistry, technology, microbiology, or other related life science
- 5-10 years of related food industry experience in Quality Assurance/Control; experience in cooking, processing, manufacturing dairy, low-acid canned food, meat, or seafood preferred
- Excellent communication and presentation skills
- Excellent research, analytical, writing, and organizational skills
Preferred
- Microsoft SharePoint® and information management systems experience
- High–risk food or ingredients experience
- Understanding of food safety in food packaging
How to Apply
Forward a resume to recruiting@goktl.com.
Company Description
KTL is a management consulting firm providing EHS, sustainability, food safety, and quality consulting services to a wide range of industry, municipal, university, and government clients. Our focus is to build strong, long-term client partnerships and provide value-added solutions that simplify management systems, improve compliance, and establish more sustainable operations. KTL specializes in developing and implementing strategies, processes, and tools that complement our clients’ investments in existing programs and resources. Our highly qualified personnel have an in-depth knowledge of U.S. federal, state, and international EHS requirements; global food safety compliance; ISO management systems; and information management tools. Our consultants possess the education, work experience, and professional registrations necessary to provide value-adding consulting services to our clients.

Comments: No Comments
Food Safety: 2024 Trends on Tap
Every year, we see a number of food safety trends rise to the surface that have the potential to impact companies across the food manufacturing and packaging industries. Some challenges and opportunities in food safety remain ongoing; some are just gaining traction with impacts yet to be known. Regardless, the start of a new year provides the opportunity to plan for food safety issues and trends on the horizon and prioritize efforts to ensure ongoing compliance.
Here are some of the top food safety trends KTL is keeping watch on in 2024—and some guidance to help you as you set your food safety strategy for the new year.
Product Reformulations
In November 2023, California became the first state in the U.S. to ban four food additives in foods sold in California through the California Food Safety Act. These additives—Red No. 3, brominated vegetable oil, potassium bromate, and propylparaben, have been linked to an array of diseases, including cancer. The rulemaking will require companies to tweak their recipes to offer products with healthier ingredients by the Act’s 2027 compliance deadline.
On the heels of California’s action, the Food and Drug Administration (FDA) is also reevaluating the use of additives, such as Red No. 3 and brominated vegetable oil. In addition, New York proposed a Bill for a similar statewide ban earlier in 2023.
Guidance: Several top brands, including Coke, Pepsi, Dunkin’ Donuts, and Panera, have taken the lead in voluntarily pulling these additives. Companies who produce foods that commonly use these additives (e.g., candy, fruit juices, packaged baked goods, and more) should be prepared for pending state and federal legislation. It is important to inventory all ingredients and to start adjusting and testing new recipes with healthier alternatives to ensure products do not have to be pulled from the shelves when compliance deadlines hit.
Food Safety Culture
Food safety culture continues to garner attention across the food industry, as it is being integrated more completely and significantly into the Global Food Safety Initiative (GFSI)-benchmarked food safety certification standards, including SQF, BRCGS, and, most recently, FSSC 22000. In fact, FSSC 22000 Version 6.0 incorporates both food safety and quality culture, requiring senior management to “establish, implement, and maintain a food safety and quality culture objective as part of the management system.” Organizations must develop and implement a documented Food Safety and Quality Culture Plan that outlines objectives and timelines and follows the management system process of continuous improvement.
Guidance: Shifting culture is a complex process that requires not just developing a Plan, but truly implementing it. Developing a robust food safety culture requires addressing how people work and what they believe. Senior leadership must prioritize food safety and quality to help create awareness, understanding, and ownership of the organization’s shared beliefs and values. Implementing robust food safety management systems (FSMS) can further help ensure consistent commitment, communication, procedures, training, performance measurement, and trust.
Food Loss and Food Waste
Wasted food makes up the largest percentage—over 20%—of any one material sent to landfills and incinerators each year in the U.S., and 58% of methane emissions released to the atmosphere from landfills are from food waste. In December 2023, the FDA, U.S. Department of Agriculture (USDA), and Environmental Protection Agency (EPA) published a Draft National Strategy for Reducing Food Loss and Waste and Recycling Organics. The Strategy defines four objectives and associated actions to meet the National Food Loss and Waste Reduction Goal of reducing food waste by 50% by 2030.
Efforts are also underway at the state level. As recent examples, New Jersey published its Recycled Content Law requiring manufacturers to meet minimum recycled content requirements for regulated containers and packaging products sold or offered for sale in New Jersey beginning on January 18, 2024. In addition, California Senate Bill 1383 further mandates a 75% reduction in organic waste disposal and requires that not less than 20% of edible food that is currently disposed be recovered for human consumption by 2025.
Guidance: GFSI standards like FSSC 22000 are now requiring organizations to develop a documented policy with objectives and detailed strategy to reduce food loss and waste within the organization and the related supply chain. A thorough food and packaging assessment serves as the foundation for these reduction efforts. Having this general understanding can help identify appropriate strategies to avoid waste, cut down on disposal costs, reduce over-purchasing and labor costs, reduce water and energy use associated with food production, and reduce greenhouse gas (GHG) emissions.
Food Traceability
The focus on supply chain management and food traceability remains a concern as companies prepare to meet the requirements of FDA’s Food Traceability Rule by January 2025. Significant efforts and investments are being made to improve traceability recordkeeping to create standardization, stronger linkages throughout the supply chain, enhanced communication, and faster response.
Guidance: Having a good document/records management system will be essential for maintaining the vast number of documents required by the Food Traceability Rule. Such a system can help ensure process and document standardization; central and secure storage, organization, and access to documents and records; enhanced workflows for approving and completing tasks involving documents; and easy access to documents for audits and clear audit trail.
Bioengineered Foods
In April 2023, FDA issued a letter to developers and manufacturers who intend to transfer genes for proteins that are known food allergens into new plant varieties for foods. The letter serves as an important reminder that developers of these new plant varieties are obligated to 1) make sure the products they market are safe for consumers; and 2) implement all measures needed to comply with the Food, Drug, and Cosmetic (FD&C) Act. If not appropriately managed, the development of these plants could result in the presence of an unexpected allergen in the bioengineered food product. And if an unexpected allergen enters the food supply, there is real risk of a severe or even life-threatening allergic reaction and, subsequently, needing to recall affected products.
Guidance: The FDA is asking developers to consider the food safety risks posed by such allergens and to plan early in development to manage those risks. Developers who intend to create these plant varieties using proteins that are food allergens need to develop a robust risk management plan that includes significantly stronger mitigation strategies and practices (e.g., crop segregation) to provide assurance that foods containing the transferred allergen are not mixed with other foods, as well as proper labeling to declare the presence of allergens.
CBD and FDA
The FDA issued a decision on January 26, 2023, concluding that “a new regulatory pathway for CBD is needed that balances individuals’ desire for access to CBD products with the regulatory oversight needed to manage risks.” FDA is concerned with the growing number of products containing CBD that are being marketed for therapeutic or medical uses without FDA approval. The Agency has sent warning letters to companies illegally selling CBD products that claim to “prevent, diagnose, treat, or cure serious diseases,” as well as to companies that sell CBD-infused food and beverages (e.g., cookies, gummies, etc.). Until a regulatory framework is established, FDA will continue to act against CBD and other cannabis-derived products to protect the public.
Guidance: Companies getting involved in this growing industry need to stay on top of the rapidly changing regulatory environment. Take the time now to assess operations, determine what emerging standards might be appropriate, identify gaps in existing programs, prepare for a new regulatory framework—state and/or federal—and begin implementing solutions to eliminate risks.
Tech Solutions
There is a common need across the food industry to manage emerging risks with solutions that also provide transparency, security, and sustainability. The recent heightened enforcement of the Foreign Supplier Verification Program (FSVP), traceability regulations, and food security concerns are pushing requirements down the supply chain and testing the entire supply chain management system. More food companies are relying on technology solutions to help manage these businesses and food safety requirements—and that will continue in 2024. Artificial Intelligence (AI) is still emerging as a business tool and could have significant impacts on how companies do business in the future.
Guidance: Having a simple, centralized FSMS to manage, track, communicate, and report compliance program information can enable staff to complete required tasks, improve compliance performance, and support operational decision-making. Companies can and should leverage existing information technology (IT) solutions to create compliance efficiencies. A well-designed and executed compliance information management system brings IT and management systems together to coordinate, organize, control, analyze, and visualize information in such a way that helps organizations remain in compliance and operate efficiently.
Other Regulatory Issues to Watch
The following Acts are all ones to watch in 2024:
- Food Labeling Modernization Act of 2023 would align labeling regulations with the latest nutrition science and advance national public health priorities through food labeling policies. The Act would require FDA to establish a standard front-of-package nutrition labeling system for all the packaged foods it regulates.
- No Toxics in Food Packaging Act of 2023 aims to prohibit certain harmful chemicals, including ortho-phthalates, PFAS, bisphenol A, styrene, and antimony trioxide, from being used in food packaging due to their cancer-causing and hormone-disrupting effects.
- Food Chemical Reassessment Act of 2023 proposes the creation of a new office within the Center of Food Safety and Applied Nutrition. The Office of Food Safety Food Assessment would reassess the safety of food additives and food contact substances.
- Transparency, Readability, Understandability, Truth, and Helpfulness (TRUTH) in Labeling Act of 2023 would require FDA to develop new front-of-package labels for foods and beverages to improve consumer access to health information.
Set Your Goals for 2024
With these challenges simultaneously competing for attention—and with fewer resources to manage it all—companies need to assess priorities, needs, and requirements and create a plan for how to meet them. KTL suggests completing the following early in 2024:
- Get senior leadership commitment and invest in creating a food safety culture that prioritizes food safety and quality.
- Understand current and pending regulatory and certification requirements and their applicability to your company. Know your operations, inventory your ingredients, understand your supply chain, quantify your food waste. All of this is necessary to help ensure compliance and sustainability.
- Seek third-party oversight. Having external experts periodically look inside your company provides an objective view of what is really going on, helps you to prepare for audits, and allows you to implement corrective/preventive actions that ensure compliance.
- Leverage IT solutions to streamline compliance, manage certification requirements, create transparency, and ensure business efficiencies.

Comments: No Comments
Food Fraud and Fish: New Guidance
Food fraud—also known as economically motivated adulteration (EMA)—occurs when someone intentionally misrepresents a less expensive food product or ingredient for a more expensive one. The Food and Drug Administration (FDA) estimates food fraud impacts about 1% of the food industry worldwide, though this number is likely higher since food fraud can be difficult to detect. Beyond its economic impacts, food fraud can cause significant health issues, ranging from lead poisoning to allergic reactions that may even result in death.
Seafood Fraud
Seafood fraud often happens when a less expensive species of fish is substituted for a more expensive species. For example, wild-caught salmon and shrimp are more expensive than farm-raised (i.e., aquacultured) salmon and shrimp. Producers who swap or mislabel fish to say it is wild caught when it is not can fraudulently earn higher profits at lower cost.
Misbranding such as this clearly results in economic fraud due to the market value of different species of fish. Of more concern, this misbranding may also prevent consumers from correctly identifying what they are eating—and the potential safety hazards associated with certain seafood. These hazards may include allergenic proteins, natural marine toxins, and scombrotoxin formation, which can all present food safety risks if the food is not accurately labeled.
This practice of mislabeling is prohibited under FDA’s Food, Drug, and Cosmetic (FD&C) Act Section 403: Misbranded Food. The Food Safety Modernization Act (FSMA) Intentional Adulteration Rule further requires companies to implement preventive controls to protect against intentional adulteration of human and animal food.
New Industry Guidance
In August 2023, FDA issued new industry guidance, including The Seafood List, to provide more information on the acceptable market, common, scientific, and vernacular names of seafood species sold in the U.S. The intent of this guidance is to:
- Help industry properly label seafood and products containing seafood ingredients with a name that is not false or misleading.
- Facilitate consistency in the U.S. marketplace.
- Reduce confusion among consumers.
In September 2023, in collaboration with the National Oceanic and Atmosphere Administration (NOAA), the National Institute of Standards and Technology (NIST) also announced four new reference materials to help assess the authenticity of seafood and verify where fish is caught or produced. These reference materials specifically address Wild-caught Coho Salmon (RM 8256), Aquacultured Coho Salmon (RM 8257), Wild-caught Shrimp (RM 8258), and Aquacultured Shrimp (RM 8259),
NIST’s reference materials are intended to help regulators and law enforcement agencies differentiate between farmed and wild-caught salmon and shrimp and assess whether imported salmon and shrimp are authentic:
- For shrimp, genetic analysis methods are used to determine the origin of the shrimp, as wild-caught shrimp are a different species than aquacultured shrimp.
- For salmon, scientists analyze the ratio of omega-3 to omega-6 fatty acids. Aquacultured salmon has roughly twice the amount of omega-3 fatty acids compared to wild-caught salmon.
Importantly, these materials can also be used for food safety purposes and detecting allergens, as well as testing for metals and other contaminants. Values for crude protein are provided in the guidance for labs to detect allergens.
According to NIST chemist Benjamin Place, “If a food processing place can use the reference material to say this is the species that it is, then consumers can have more confidence. You now know when you go to a store, you can have full faith the seafood product is the species it says it is and that the labels are true.”
What You Can Do
As always, it is better to take a proactive approach to managing food fraud rather than being caught on the defensive. Facilities should:
- Conduct a vulnerability analysis to identify those areas that pose the greatest risk of food fraud or intentional adulteration.
- Develop and implement a Food Defense Plan to outline risks, mitigation/prevention strategies, monitoring plans, corrective action response, verification activities, and recordkeeping policies.
- Train employees to understand what food fraud is, how to identify fraudulent products specific to their work, and their responsibilities in ensuring food safety by reporting suspicious materials.
- Conduct a mock exercise to assess the effectiveness of the Food Defense Plan and intentional adulteration programs.

Comments: No Comments
Food Allergen Programs: Updated FDA Draft Guidance
According to the Food and Drug Administration (FDA), millions of Americans have food allergies. While most reactions are mild, some people experience severe or even life-threatening symptoms. FDA’s Current Good Manufacturing Practice (cGMP), Hazard Analysis, and Risk-Based Preventive Controls for Human Food (PCHF) Rule (21 CFR Part 117) requires domestic and foreign food facilities that manufacture human food develop and implement a Food Safety Plan that includes an Allergen Program to address this concern.
Eight Becomes Nine
While many different foods can cause many different types of reactions and symptoms, the Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) identified what has commonly been known as the “Big 8” major allergens:
- Milk
- Eggs
- Fish (e.g., bass, flounder, or cod)
- Crustacean shellfish (e.g., crab, lobster, or shrimp)
- Tree nuts (e.g., almonds, pecans, or walnuts)
- Wheat
- Peanuts
- Soybeans
Sesame was added as the ninth major food allergen when the Food Allergy Safety, Treatment, Education, and Research (FASTER) Act became effective on January 1, 2023. FALCPA requires that food labels clearly identify the food source names of any ingredients that are a major food allergen or contain protein derived from a major food allergen. The intent of FALCPA is to help allergic consumers identify foods or ingredients that they should avoid to prevent an allergic or other reaction due to hypersensitivities.
New Guidance
On the heels of the FASTER Act’s effective date, FDA recently published draft guidance for Food Allergen Programs in September 2023. This is part of the Agency’s ongoing updates to its Draft Hazard Analysis and Risk-Based Preventive Controls for Human Foods Guidance for Industry. Comprised of 16 chapters, FDA has been releasing new chapters since the guidance was announced in 2016 as they are developed to help facilities develop a Food Safety Plan in accordance with regulatory requirements. The most recent guidance published includes Chapter 11: Food Allergen Program.
Chapter 11 focuses on developing a Food Allergen Program to ensure finished food is properly labeled for major food allergens and, even more so, to protect food from major food allergen cross-contamination. According to FDA, “Some manufacturers are intentionally adding sesame to products that previously did not contain sesame and are labeling the products to indicate presence, rather than taking appropriate measures to minimize or prevent cross-contact.” Through the new guidance, FDA is encouraging industry to prevent allergen cross-contamination rather than intentionally adding sesame (or other major allergens) to products and then labeling them to comply with the law.
FDA recognizes the challenges of ensuring products are allergen-free, and the guidance is intended to help companies find solutions to meet the needs of consumers with food allergies. Chapter 11 explains how to develop and implement a Food Allergen Program by providing detailed recommendations for each aspect of the Program. It offers multiple examples for illustrative purposes that demonstrate ways to significantly minimize allergen cross-contact and undeclared allergens using cGMPs and preventive controls.
Labeling errors are the major reason for most FDA food allergen recalls. As such, the new chapter also offers guidance on how to monitor and verify that food allergies are properly declared and correctly labeled. In addition, the guidance addresses what facilities can do when allergen presence due to cross-contact cannot be completely avoided, including using allergen advisory statements.
Previously Published Guidance
The comprehensive Draft Guidance for Industry remains a work in progress. In addition to Chapter 11: Food Allergen Programs, the following draft chapters are currently available:
- Chapter 1: The Food Safety Plan
- Chapter 2: Conducting a Hazard Analysis
- Chapter 3: Potential Hazards Associated with the Manufacturing, Processing, Packing, and Holding of Human Food
- Chapter 4: Preventive Controls
- Chapter 5: Application of Preventive Controls and Preventive Control Management Components
- Chapter 6: Use of Heat Treatments as a Process Control
- Chapter 14: Recall Plan
- Chapter 15: Supply-Chain Program for Human Food Products
- Chapter 16: Acidified Foods
FDA plans to still publish the following chapters as they are developed:
- Chapter 7: Use of Time/Temperature as a Process Control
- Chapter 8: Use of Product Formulation or Drying/Dehydrating as a Process Control for Biological Hazards
- Chapter 9: Validation of a Process Control for a Bacterial Pathogen
- Chapter 10: Sanitation Controls
- Chapter 12: Preventive Controls for Chemical Hazards
- Chapter 13: Preventive Controls for Physical Hazards
- Chapter 17: Classification of Food as Ready to Eat or Not Ready to Eat
Whether for allergen management or other identified hazards, taking a systematic approach to establishing risk-based preventive controls helps protect food products—and the consumer—from biological, chemical, and physical risks. FDA’s guidance can provide an excellent resource for meeting regulatory requirements and protecting consumers.
Comments: No Comments
Food Safety Certification: Top Non-Conformances
In July, the Safe Quality Food (SQF) Institute published an article citing the most common non-conformances encountered during certification audits. Interestingly, the transition to SQF edition 9 has changed the number and types (i.e., critical, major, minor) of non-conformances SQF is seeing, with non-conformances related to pest prevention, Food Safety Plan, cleaning and sanitation, and management review and internal audits topping the list.
KTL’s food safety experts break down SQF’s top non-conformances and what you can do to address them.
Prioritizing Pest Prevention
According to SQF, pest prevention is the leading non-conformance under edition 9, with both critical and major findings. Exposure to pests—and the diseases they carry—creates the risk of food contamination and the spread of infectious diseases. Companies need a pest prevention program, whether managed internally or by a third-party contractor, that integrates sufficient measures to minimize pest populations. This may include mechanical preventions and controls, waste minimization, or controlled use of pesticides.
KTL Recommendations:
- Review any findings with your food safety team and the pest control contractor, if used. Include frequent review of the approved chemicals or chemicals used for any treatment and ensure the site has access to copies of their safety data sheets (SDSs). Corrective and preventive actions (CAPAs) should be applied to act right away on any open observations. CAPAs should be regularly monitored and tracked to closure. If a third-party contractor is used, open observations should be discussed with the contractor; they may have helpful information on how to handle a pest issue.
- Review pest control trending data at monthly management review meetings. This will help ensure pest prevention data is tracked and monitored. It can also help identify larger, more systemic issues that might necessitate additional CAPAs to resolve the problems.
- Incorporate evaluation of pest prevention performance into the validation and verification schedule.
- Validation should include review of inspection records (at each visit), an annual assessment by the food safety team or pest control provider, and review of trends at the annual management review meeting.
- Verification should include a monthly visual inspection during internal good management practices (GMP) inspections. A simple check to ensure these inspections are thorough enough can involve putting a business card in a tin can for pest control to find and identify on the report.
SQF Tip Sheet: Pest Prevention
Strengthening the Food Safety Plan
The Hazard Analysis and Critical Control Points (HACCP) Food Safety Plan is the foundation of the SQF System. Given this, it makes sense that non-conformances related to the Food Safety Plan always top the list. SQF indicates that many of these findings are now being marked as critical with edition 9. The most common non-conformances include missing hazard analysis, incomplete ingredient hazard analysis, and misidentification of critical control points (CCPs).
KTL Recommendations:
- Review and update the HACCP Plan at least annually or whenever there is a change to operations (e.g., ingredients, processes, equipment, etc.) to ensure all inputs and outputs are identified and appropriately managed.
- Use a risk ranking chart to identify risks; determine their severity and likelihood; and document when a hazard is controlled by a GMP, CCP, or other preventive control (PC).
- Use specification sheets and known information about ingredients to facilitate the identification of hazards during the hazard analysis. Ensure copies of any studies or guidance documents are available to the HACCP team and any applicable updated scientific consensus is reviewed. Hazards should be specifically identified rather than just listed as general categories (e.g., biological, chemical, etc.).
- Document everything in a food safety management system (FSMS). Recordkeeping proves that all requirements of the Plan are met.
SQF Tip Sheet: HACCP Food Safety Plan
Emphasizing Cleaning and Sanitation
Cleaning and sanitation methods vary based on the nature of operations, as well as the microbiological and allergen risks. Regardless, every facility needs to develop, implement, and document a cleaning and sanitation program that fits their production processes. According to SQF, this area remains second on the list of major findings in edition 9.
KTL Recommendations:
- Understand all the areas and equipment that need to be cleaned and sanitized in the facility. Pay attention to the condition of floors, ceilings, walls, doors, etc. to ensure you are maintaining a hygienic and safe environment.
- Create a robust cleaning and sanitation, preventive maintenance, and maintenance schedule. Regular maintenance of equipment, utensils, and building materials is crucial to prevent non-conformances.
- Incorporate monitoring of the cleaning and sanitation program into the validation and verification process:
- Validation: Review sanitation records and logs, environmental monitoring records, and trending data to identify areas of concern.
- Verification: Conduct visual inspections and records review. Consider swab testing to monitor compliance and trends, especially if using a contracted company for cleaning and sanitation. Review GMP inspection findings and CAPAs at monthly management meetings and track to closure.
SQF Tip Sheet: Cleaning and Sanitation
Management Review and Internal Audits
SQF includes management review (2.1.2.1) as minor nonconformance, highlighting the importance of incorporating food safety culture into the review process. Internal audits remain a crucial way to identify areas of improvement, though they are now on the minor non-conformance list.
KTL Recommendations:
- Set objectives/goals for the year and monitor the performance of these objectives at monthly and annual management review meeting. Resources should be allocated appropriately based on trends identified in these meetings to reach goals and ensure the overall effectiveness of the food safety culture.
- Conduct regular GMP inspections, trend results, review them during management meetings, and identify CAPAs, as necessary.
- Distribute food safety questionnaires to personnel to gather input. Review results during management review meetings and use the data collected to create action plans for maintaining or improving scores.
- Conduct a comprehensive internal audit at least annually and address any identified gaps in compliance. Internal audit findings should be reviewed at monthly and annual management review meetings. CAPAs should be assigned for findings, and data can be used to refine food safety objectives/goals.
SQF Tip Sheets: Management Commitment / Internal Audit Plan
Knowing and truly understanding the requirements of the SQF Code—or any of the Global Food Safety Initiative (GFSI) certification standards—is essential to avoiding non-conformances. Paying particular attention to the identified top non-conformances can help facilities to proactively mitigate these risks, strengthen food safety culture, and improve overall compliance.
